INTRODUCTION
1) COPD
- Chronic Obstructive Respiratory Disease (COPD) is
characterized by persistent airflow obstruction, resulting from inflammation
and remodelling of the airways, and may include development of emphysema.
- Extra-pulmonary degenerative manifestations that may occur
in COPD include osteoporosis and muscle wasting.
2) Muscle wasting in
COPD
- The prevalence of muscle wasting is relatively high in
COPD: 15–40% depending on definition and disease stage.
- Importantly, muscle wasting not only contributes to
diminished skeletal muscle function, reduced exercise capacity, and decreased health
status, but is also a determinant of mortality
in COPD, independent of airflow obstruction
- Muscle wasting in COPD has been demonstrated by decreases
in fat-free mass (FFM) at whole body level, but also specifically at the level
of the extremities
- Muscle wasting is apparent as a decrease in the size of
individual muscle fibres, and this muscle fibre atrophy in COPD seems selective
for type II fibres in peripheral muscle, which is in line with other chronic
diseases prone to cachexia such as chronic heart failure
- A shift in muscle fibre composition from type I
(oxidative) to type II (glycolytic), accompanied by a decrease in oxidative capacity,
culminates in reduced muscle endurance.
- This not only contributes to reduced exercise capacity but may also affect muscle mass in COPD, because type I and II fibres display
different responses to anabolic and catabolic signals
3) Unintended weight
loss in COPD
- There is now convincing evidence that unintended weight
loss is an independent determinant of
survival, arguing for weight
maintenance in patient care
- There are indications that the pathophysiology of
unintended weight loss is different between clinically stable COPD and during
acute flare-ups of the disease.
- To date, data in acute exacerbations of COPD are, however,
very limited. Therefore, lung cancer is used as a comparative acute pulmonary
cachexia model
- A recent unbiased statistical approach suggests that not
all COPD patients but only the emphysematous phenotype is prone to cachexia, although
the informative value of available clinical studies is limited by a
cross-sectional study design
IDENTIFYING MUSCLE
WASTING IN COPD
- Traditionally, reference values for fat-free mass index (FFMI)
in COPD were developed based on age-specific and gender-specific 10th
percentile values
- The recent European Respiratory Society statement on
nutritional assessment and therapy in COPD proposed dual-energy X-ray
absorptiometry (DEXA) as the most
appropriate method for body composition analysis in COPD, mostly because it combines
screening for osteoporosis with assessment of fat mass (FM) and fat-free mass (FFM)
at the regional level in addition to whole body level.
-Body composition assessed by DEXA also allows measurement
of appendicular skeletal muscle mass (ASM), which has been demonstrated to be
stronger related to physical functioning than total FFM.
NEW INSIGHTS IN THE
PATHOPHYSIOLOGY OF MUSCLE WASTING IN COPD
- Triggers of muscle wasting include hypoxemia, oxidative
stress, inflammation, impaired growth factor signalling, oral glucocorticoids,
disuse, and malnutrition, some of which are influenced by smoking
- Wasting of skeletal muscle is due to a net catabolic
state, which may result from an imbalance in muscle protein synthesis and breakdown
(protein turnover), as well as from an imbalance in myonuclear accretion and
loss (myonuclear turnover).
1) Protein turnover
- Both increased and normal rates of whole body protein
turnover have been reported in patients with COPD, but the relative
contribution of muscle versus other tissues to protein turnover is unknown
- Rutten et al. observed an increase in myofibrillar protein breakdown in cachectic COPD
patients compared with non-cachectic patients and controls, but no data are
available regarding muscle protein synthesis rate, except for a small study
showing depressed muscle protein synthesis rates in malnourished patients with
emphysema.
2) Proteolytic
signalling
- Several environmental triggers can lead to catabolic
signalling in the skeletal muscle, mediated by transcriptional regulators including
nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB) and
forkhead box O transcription factors (FOXOs).
- Increased catabolic signalling through FOXO and NF-κB can induce
gene expression of key factors in both the ubiquitin proteasome system (UPS) and
the autophagy lysosome pathway
- The respiratory muscles of COPD patients show an opposite fibre
type shift compared with limb muscles, that is, towards more type I fibres. This
will have implications for the expression levels of constituents of atrophy
signalling pathways.
3) Ubiquitin
proteasome-mediated degradation
- The majority of the literature suggests that wasting in COPD
is accompanied by an increase in UPS activation.
- The increase in
catabolic signalling in cachectic COPD patients is site specific.
- This may reflect disuse atrophy of the limb muscle with
maintained or increased respiratory muscle activity, or it may result from an
interaction between inactivity and other triggers of atrophy, such as smoking.
4) Autophagy-lysosome-mediated
degradation
- The autophagy-lysosome pathway is a protein degradation pathway.
- Upon activation, autophagosomes form and mature to
subsequently fuse with lysosomes. The autophago-lysosomes degrade the cargo and
release amino-acids for de novo protein synthesis or other metabolic fates
- It currently is unknown
if the autophagic-lysosome pathway activity is altered during acute
exacerbations of COPD, because most studies were conducted in stable COPD patients.
- However, in lung cancer cachexia, markers of increased in
autophagy was observed. From this, autophagy induction in skeletal muscle might
be anticipated during acute stages of COPD wasting
5) Protein synthesis
signalling
- A major anabolic pathway is the IGF-1/PI3K/AKT pathway
- Studies show an increase in protein synthesis signalling
in the limb muscles of cachectic
COPD patients compared with non-cachectic COPD patients, but
no alteration in the general COPD population.
- Only limited data are available on anabolic signalling in respiratory
muscles of COPD patients, and although the results also point to an increase in
anabolic signalling, it remains unclear if this is different between cachectic
and non-cachectic COPD patients
- Taken together, anabolic
signalling is increased in the skeletal muscle of patients with COPD, with
an even larger increase in the diaphragm than the limb muscles.
- One may speculate that the increased activation of AKT
signalling in the respiratory muscles is an attempt to preserve respiratory
function by compensating catabolic triggers, although it may also reflect
intrinsic alterations in muscle fibre composition.
6) Myonuclear
turnover
- Besides the turnover of proteins, the turnover of
myonuclei appears essential for muscle regeneration. Furthermore, although at a
lower rate, myonuclear turnover might be indispensable for the maintenance of
skeletal muscle mass.
- To gain further insight in the regulation of myonuclear
turnover and possible defects in COPD-induced skeletal muscle wasting, it is
essential to incorporate satellite cell activation stimuli and sensitive
techniques to monitor myonuclear accretion and turnover in the study design
7) Loss of muscle
oxidative phenotype
- Besides the importance of the muscle quantity for muscle function,
the quality of the muscle should
also be considered.
- It was found that muscle mass-specific muscle strength and
endurance are reduced in patients with COPD
- A well-established qualitative alteration in the skeletal
muscle of COPD patients is the loss of oxidative phenotype (OXPHEN)
characterized by a muscle fibre type I to type II shift and a loss of oxidative
capacity
- The loss of OXPHEN is associated with increased oxidative
stress, which may render the muscle more susceptible to muscle atrophy In
addition, type II fibres are generally more susceptible to atrophy stimuli
including, for example, inflammation and hypoxia. Therefore, the loss of OXPHEN in COPD may accelerate the
loss of muscle mass, thereby linking muscle quality to muscle quantity.
8) Therapeutic
perspective
- Pharmacological inhibitors that target specific ubiquitin-conjugating
and deconjugating enzymes are being developed to treat cancer,
neurodegenerative disorders, and autoimmune diseases but may also be highly
relevant for the treatment of COPD-induced muscle wasting.
- So far, exercise seems
to be the only intervention that can target UPS and autophagy leading to
improved quantity, as well as an improved quality of the muscle in COPD patients.
- One prerequisite is that COPD patients, and specifically
cachectic COPD patients, have maintained responsiveness to exercise stimuli,
which remains to be established.
- Exercise capacity in COPD may be limited by impaired
pulmonary function, leading to incapability to supply a sufficiently strong
exercise trigger to the muscles. In this case, pharmacological or nutritional activators
of AMPK, sirtuin 1, and peroxisome proliferator-activated receptors such as
metformin, resveratrol, rosglitazone, and polyunsaturated fatty acids could be used
as exercise mimetics and may help sensitize the muscle to a following exercise
bout.
- It should also be considered that an appropriate nutritional status is necessary for the beneficial effects
of exercise and that exercise (in particular, endurance type of exercise)
in a malnourished state could even have detrimental effects by worsening the
energy imbalance
PUTATIVE MECHANISMS
INVOLVED IN A DISTURBED ENERGY BALANCE IN COPD
- Specific loss of muscle mass in weight-stable COPD
patients has been observed, which may reflect a tissue-specific sensitivity to
an overall catabolic state
- A net catabolic state may also result from an imbalance in
energy expenditure and energy availability (energy balance).
1) Increased energy
expenditure
- Numerous studies have shown that REE is raised.
- This is more prevalent in emphysema during acute exacerbations,
and appears inversely correlated with forced expiratory volume in 1 s when
comparing different studies
- Highest values
are found among weight-losing patients;
this is in contrast with
non-pathology-induced malnutrition,
where subjects with low BMI have lower REE due to hypometabolic adjustments
- Activity-induced energy expenditure is the most variable component
of TEE, and it has been postulated that COPD patients reduce physical activity
to compensate for dyspnoea severity or to anticipate to breathlessness
- There are several indications that when COPD patients
perform physical activities, they require more energy- may indicate that COPD patients
use oxygen less efficiently and
exhibit an altered energy metabolism during physical activity
- The thermic effect of dietary intake remains unclear.
2) Adipose tissue
metabolism
- In COPD, low BMI and fat mass depletion particularly occur
in those with advanced disease and in the emphysematous phenotype
- There is some indirect evidence pointing towards a role of
brown adipose tissue in pulmonary cachexia, but this area requires more
research to identify therapeutic potential.
COMPROMISED DIETARY
INTAKE
- In order to compensate for increased energy requirements
in COPD, patients should be able to adapt their dietary intake
- In terms of caloric content, dietary intake was found to
be normal compared with healthy controls, but inadequate for measured energy
expenditure
- During severe acute exacerbations, the gap between energy
intake and energy expenditure becomes even wider, which slowly decreases upon
recovery.
1) Anorexia
- A few underlying causes have been mentioned, including nicotine use, physical discomfort such
as dyspnoea and increased breathing effort,
depression, and anxiety, seen in COPD as well as in non-small cell lung cancer.
- Besides pulmonary and psychological symptoms, COPD patients
often experience pain Opioids are
commonly used to combat pain in COPD. Side-effects of opioids occur regularly,
and opioids are able to cause gastrointestinal motility disorders, of which constipation is the most common
- Separate from use of pain medication, early satiety and abdominal
bloating is highly prevalent in COPD.
2) Chemosensory
alterations
- Food intake is regulated by taste and smell, and chemosensory
dysfunction could influence dietary intake.
- Reduced smell and
taste test scores was found among COPD patients compared with controls,
independent of oxygen supply.
3) Food reward system
- Fullness is regulated by gastrointestinal hormones,
including leptin (↓ food
intake, ↑ energy expenditure) and ghrelin (↑ food intake), and their secretion is affected by dietary
intake and nutritional status.
- Clinically stable emphysematous COPD patients exhibit low
leptin levels compared with the chronic bronchitis subtype
- Brain imaging studies have revealed reward-specific brain
regions related to food reward, and activation of these regions correlate with
food rewarding. However, there is surprisingly no human study available that explored
the role of central dysregulation in food reward in patients with COPD.
4) Therapeutic
perspective
- The importance of nutritional status is not only
emphasized by adverse effects on muscle function and exercise performance but
also by detrimental effects of malnutrition on lung tissue.
- Efthimiou et al in their RCT found that nutritional support among malnourished
COPD patients improved muscle strength and hand grip strength, accompanied by
less dyspnoea and enhanced distance in 6-min walk test. These effects diminished
after quitting the dietary supplementation.
- Weekes et al demonstrated weight gain in the intervention group
with dietary support while the
control group continued to lose weight. Addition of dietary counselling to dietary support has been shown to maintain
weight loss after cessation of intervention
- Besides energy,
optimal protein intake is also very
important.
- Low intake of other essential nutrients is identified,
including vitamin D and calcium, which are also relevant in the context of
osteoporosis as clustering comorbid condition
- One should keep in mind that dietary intake does not reflect
actual availability of ingested micronutrients. There are indications that
intestinal function is impaired in COPD, illustrated by splanchnic
hypoperfusion and reduced intestinal permeability
- Ghrelin analogues warrant further investigation in COPD.
- Cognitive behavioural interventions are relatively underexplored
in the management of cachexia in COPD
CONCLUSIONS
- In order to increase overall survival and compress
morbidity, a multi-modal intervention approach is needed, which should target
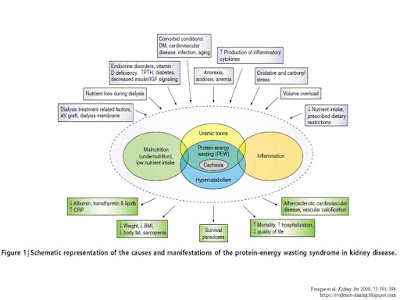
the discussed factors involved in cachexia (Figure 1).
- Such a multi-modal intervention approach, encompassing
exercise training and improvement of energy balance and nutrient availability,
is currently feasible as supported by recent statements and meta-analyses,
possibly improved in the near future by targeted pharmacological interventions
and cognitive behavioural therapy to sensitize patients to anabolic stimuli
Further reading: