INTRODUCTION
- Malnutrition is one of the most common complications
occurring in patients with advanced liver disease, regardless of the etiology,
and has important prognostic implications.
- It is estimated that malnutrition is present in at least
50% and up to 90% of patients with cirrhosis
- Malnutrition appears to be more common in those patients
hospitalized with alcoholic liver disease than nonalcoholic liver disease.
- Malnutrition, when present in cirrhotics, is associated
with an increased risk for morbidity, mortality, compromised immune function,
respiratory function, decreased muscle mass, increased recovery time, and
delayed wound healing
- The presence of ascites and hepatorenal syndrome is
significantly greater among those patients with concurrent protein-calorie
malnutrition (PCM)
The progress from
compensated to decompensated cirrhosis
- Advanced liver disease, although typically progressive,
may present with a long initial period of well-compensated cirrhosis.
- Compensated cirrhosis occurs when the liver is heavily
scarred but is still able to perform many of its important functions.
- Individuals with compensated cirrhosis often have few or
no symptoms; however, liver decompensation may occur rapidly and dramatically.
- Decompensated cirrhosis occurs when the liver is
extensively scarred and unable to function properly and is often accompanied by
complications (eg, ascites, variceal bleeding, encephalopathy) that can be
life-threatening
- With the high prevalence of malnutrition and its
associated poor outcomes in patients with advanced liver disease, it is crucial
to identify malnutrition early and initiate aggressive nutrition interventions
to prevent further complications and decrease hospital length of stay
CAUSES OF
MALNUTRITION
- The clinical conditions and pathophysiological mechanisms that
drive cirrhotic patients to a disruptive metabolic state are multiple and
interrelated.
-Major contributors to malnutrition include inadequate oral intake, metabolic disturbances, malabsorption, and decreased capacity of the liver to store nutrients.
- Table 1
provides a list of potential causes of the malnutrition seen in advanced liver
disease
NUTRITION
ASSESSMENT
- All patients with advanced liver disease should undergo nutrition screening to assess risk of
developing malnutrition.
- Nutrition screening is the initial step in the
documentation of nutrition care, and it can be performed by any healthcare
professional in either an inpatient or an outpatient setting
- Nutrition
assessment is more comprehensive than screening and is defined by the American
Society for Parenteral and Enteral Nutrition (A.S.P.E.N.) as “a comprehensive approach to define
nutrition status that uses medical, nutrition, and social histories; physical
examination; anthropometric measurements; and laboratory data”.
- A properly conducted nutrition assessment provides a
global assessment of both nutrition
status and the severity of underlying illness—both important considerations given the fundamental
relationship between the two.
1) Assessment of
Nutrition History and Energy Intake
- Review nutrition history to assess intake of energy, protein,
macro- and micronutrients, and overall understanding of and compliance with the
prescribed diet
- Note factors contributing to diet history such as past
dietary restrictions, appetite, satiety levels, taste changes, socioeconomic
status, fad diets, supplement use, ethnic or religious preferences, and food intolerances
or allergies.
- Although all have limitations, oral intake history may be
obtained using the 24-hour diet recall, food frequency questionnaire, calorie
counts, or food diary
- Nutrition risk can be predicted using the Subjective Global
Assessment (SGA) scale, which is a score based on medical diagnoses, weight
changes, intake, gastrointestinal (GI) symptoms, and function capacity and
physical signs of malnutrition.
- Table 2 lists
available nutrition assessment tools and their respective advantages and
limitations
2) Assessment of Body
Composition
- Individuals with cirrhosis have been shown to have
increased extracellular and decreased intracellular fluid compared with healthy
individuals
- A decrease in muscle mass and adipose tissue is often
present but not always apparent as it may be masked by fluid retention
- Peng et al: men lost an average of 20% of their body
protein stores compared with only 11% in women; patients with higher protein
depletion also had significantly decreased bone density, hand grip strength,
and respiratory muscle strength.
- Changes in body composition have been shown to progress with
the course of the liver disease and correlate well with the Child-Pugh score, a
commonly used method for assessing the severity and prognosis of liver disease
- The Child-Pugh score is calculated using lab values such
as serum albumin and bilirubin, as well as factors such as the presence of
ascites and encephalopathy.
- Child-Pugh score A has the best prognosis of survival of
100% at 1 year, whereas a B or C score carries a prognosis of 81% and 45% at 1
year, respectively
- Physical examination should include an assessment of musculature, adipose tissue,
body habitus, oral cavity, skin, hair, nails, and temperature
- In the cirrhotic
patient, special attention should
be paid to the presence of muscle
atrophy, ascites, and peripheral edema
- It is often difficult to accurately identify malnutrition
and body composition changes in cirrhotic patients who have ascites and edema,
as fluid weight gains mask muscle and adipose tissue losses
- The visualization
of muscle wasting may be more
evident in the temporal, clavicular,
and scapular regions, as they are typically less affected by fluid retention
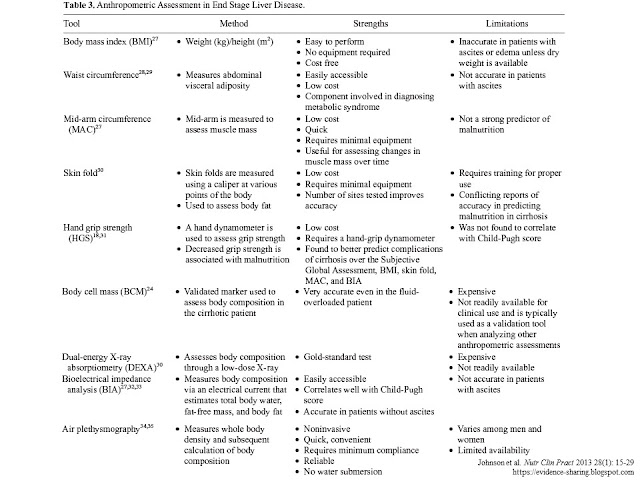
- Tools available to aid in the assessment of body composition
include skin-fold measurement, dual-energy X-ray absorptiometry scan (DEXA),
handgrip strength (HGS), body cell mass (BCM), and bioelectrical impedance
(BIA); however, some of these tools may not be readily available to the
clinician, and their accuracy may be limited in the presence of fluid
retention.
- Table 3 reviews
available tools for assessing body composition in the cirrhotic patient,
including their strengths and limitations.
3) Biochemical
Assessment
- Prealbumin (transthyretin) and serum albumin are produced
in the liver and are commonly, yet erroneously, considered indicators of
nutrition status.
- Due to the location of their synthesis and response to
inflammation, the value of prealbumin and serum albumin as indicators of
nutrition status is poor, particularly in patients with advanced liver disease.
- Although prealbumin and serum albumin may not be accurate
nutrition indicators, they appear to have use as markers of prognosis,
morbidity, and mortality
- As liver disease progresses, the synthesis of prealbumin
and serum albumin is reduced, a property that has historically made them useful
as markers of liver disease severity
- Therefore, prealbumin, serum albumin, and transferrin
reflect the severity of underlying illness and inflammation and not the
individual’s nutrition status.
MICRONUTRIENTS
- Micronutrient
deficiencies are common in end-stage
liver disease.
- Therefore, periodic assessment of serum vitamin levels is
important to allow for appropriate supplementation to prevent complications
related to deficiencies.
- Table 4
summarizes the more common micronutrient deficiencies that may occur in liver
disease, the role these micronutrients play in liver disease, the signs of
micronutrient deficiency, and recommended supplementation.
1) Fat-soluble
vitamins
- Deficiencies in fat-soluble vitamins (A, D, E, and K) are
likely to develop in liver disease due to reduced oral intake, malabsorption,
and/or hepatic effects involving reduced synthesis of carrier and transfer
proteins.
- It has been demonstrated that as the severity of liver
disease progresses, fat-soluble vitamins tend to become deficient
- Vitamin A
deficiency may cause nyctalopia
(ie, night blindness) and dry corneas
and is associated with increased risk for the development or cirrhotic
progression to hepatocellular cancer in patients with end-stage liver disease.
- Retinol is typically normal in Child-Pugh score A patients
and decreased in Child-Pugh score B and C
- Suboptimal vitamin
D stores can be associated with both cholestatic and noncholestatic liver
disease
- More than 90% of those with advanced liver disease are
vitamin D deficient. Patients who have progressed to cirrhosis have twice the
incidence of deficiency as their noncirrhotic counterparts.
- Depending on the etiology of liver disease, deficiency may
be attributed to low vitamin D intake,
fat malabsorption, bile acid deficiency, and impaired liver function
- Vitamin D deficiency
in adults contributes to the development of osteopenia and osteoporosis
and may cause osteomalacia, bone pain,
and muscle weakness.
- Vitamin D supplementation is recommended in all patients with
chronic liver disease in combination with calcium.
- Oral replacement
with vitamin D2 or vitamin D3 (2000 IU)
daily is generally adequate along
with 1200–1500
mg/d of calcium.
- Vitamin E
levels are low in nearly half of cirrhotic patients.
- Clinical manifestations of vitamin E deficiency may include increased platelet aggregation, decreased red blood cell survival, hemolytic
anemia, decreased serum creatinine with creatinuria, and neuronal degeneration
- It has been theorized that administration of vitamin E may
ameliorate oxidative stress. Oxidative stress may initiate cellular injury,
leading to a chronic inflammatory response. Vitamin E may act as a
chain-breaking antioxidant that neutralizes free radicals.
- Low vitamin E levels
have also been linked to high triglyceride levels, and it has been
suggested that deficiency of this vitamin may lead to enhanced progression of fatty liver to steatohepatitis
- A dosage ranging from 400-1200
IU daily in nonalcoholic steatohepatitis (NASH) was shown to improve serum
aminotransferase levels and fibrosis scores
- Also, steatosis
was also improved with vitamin E
supplementation ranging from 300 to 1000
IU daily for 6 months to 3 years
- Vitamin K is an
important clotting factor but also plays a role in skeletal development and
bone health
- The most common sign of deficiency is bleeding.
- Patients with vitamin K deficiency tended to have elevated
serum alkaline phosphatase and bilirubin levels and more advanced histological
staging compared with patients with normal vitamin K levels.
- For patients with impaired prothrombin time (and/or
international normalization ratio [INR]), vitamin K supplementation should be considered
2) Water-soluble
vitamins
- Symptoms of a neuropsychiatric nature seen in patients
with chronic liver disease may be the consequence of water-soluble vitamin
deficiencies
- Deficiency in pyridoxine,
thiamine, or vitamin B12 may result in peripheral neuropathy and other
neurologic disturbances
- Due to diminished hepatic storage, deficiencies in
thiamine and folic acid may develop more rapidly in cirrhotic patients than
those without liver disease.
- In addition to liver disease causing pyridoxine
deficiency, inadequate diet, alcoholism, medications, and chronic renal failure
can also contribute to deficiency
- Serum B12 level
tends to be falsely elevated in patients with cirrhosis because the
laboratory value includes endogenous metabolically inactive cobalamin analogues
(transcobalamins I and III), despite vitamin B12 tissue levels being depleted
- Ocular disturbances,
confusion, and ataxia are classical signs of thiamine deficiency and have
been reported in patients with alcohol-related and chronic hepatitis C-related
cirrhosis
- Individuals with chronic
alcohol-related liver disease, particularly when accompanied by mental
status changes, should receive thiamine
supplementation due to the high risk of Wernicke
encephalopathy (WE).
3) Trace elements
- Zn deficiency
in cirrhotic patients may lead to anorexia,
altered taste and smell, immune dysfunction, and altered protein metabolism.
Other manifestations of Zn deficiency may include skin rash, depressed mental function, hypogonadism, poor wound healing,
and altered immune function
- Serum Zn is bound
to serum albumin, which is typically decreased in advanced liver disease;
therefore, caution is needed regarding overinterpretation of serum Zn levels.
Zinc deficiency in liver disease may be related to increased losses in the
gastrointestinal system due to diarrhea or intestinal malabsorption, increased
urinary losses and reluctance regarding protein intake
- Despite conflicting data on oral Zn supplementation and
its effects on hepatic encephalopathy, improved Zn status has been associated
with improvement in liver function. Zinc may be used long-term or at least
until the serum zinc level has normalized. A once-daily dose of 50 mg elemental zinc (220 mg zinc sulfate) is
recommended which does not inhibit
copper absorption
- If malabsorption is the cause of malnutrition in alcoholic
and cholestatic liver disease, consider the use of medium-chain triglycerides
(MCT) and pancreatic enzymes
- Concentrations of homocysteine, cysteine, copper (Cu), and
the Cu:Zn ratio in cirrhotic patients were found to be significantly higher
compared with healthy individuals
- Furthermore, the Cu:Zn ratio was shown to be significantly
lower as liver disease severity progressed according to the Child-Pugh score.
- Copper and
manganese are both excreted in the bile, and it is recommended that both of these be decreased or omitted from parenteral
nutrition (PN) formulations in patients with cirrhosis and/or cholestasis
NUTRITION MANAGEMENT
- Goal: to meet
estimated energy needs and to prevent further protein catabolism.
- This may be accomplished via oral, enteral, parenteral, or
a combination of these routes.
Estimating Protein and Energy Requirements
- Experts in the
field agree that compensated, stable patients have energy requirements near
normal, with more critically ill patients having higher requirements.
- For well-compensated patients, 25-35 kcal/kg/d of energy is
appropriate.
- Malnourished patients may require 30-40 kcal/kg/d to promote anabolism
- Historically,
protein restriction was recommended in advanced liver disease to prevent an
increase in serum ammonia level and reduce the risk of hepatic encephalopathy
- Recent studies,
however, have shown that high-protein diets are more beneficial in cirrhosis
with respect to prognosis due to improvement in overall nutrition status
without exacerbating hepatic encephalopathy
- Cordoba et al in
a RCT showed that restriction of protein actually worsens hepatic
encephalopathy due to increased protein catabolism, and a protein intake of 0.5
g/kg/d led to increased muscle breakdown compared with an intake of 1.2 g/kg of
protein per day
- It is now
recommended that patients with cirrhosis
consume 1.0-1.5 g/kg of protein per day
to prevent muscle catabolism and promote gluconeogenesis.
- A temporary protein restriction (0.6-0.8
g/kg/d) may still be appropriate in patients with acute exacerbations of
encephalopathy until the cause is determined and eliminated, at which time a high protein intake can be
resumed.
- Table 5 lists methods for determining
energy and protein requirements relevant to the patient with advanced liver disease.
- Note that a dry weight or estimated dry weight
should be used when calculating energy and protein needs
Carbohydrate and Fat Requirements
- Many patients
with cirrhosis have glucose intolerance or frank diabetes. Glucose intolerance
has been found to correlate with poor prognosis of patients with cirrhosis.
- Glucose should not be given in doses larger than 5-6 g/kg/d, and serum glucose should
be monitored closely
- Impaired fat metabolism is present in cirrhosis with
incomplete metabolism of long-chain triglycerides.
- Overfeeding, regardless
of the energy source, should be avoided
because excess calories can contribute to fat synthesis and accumulation in the
liver.
- A range of 25%-30%
of the calories from fat is generally recommended.
- If steatorrhea
is present, medium-chain triglycerides
may be useful to supplement a low-fat diet
Fluid needs
- Fluid intake of 30-40
mL/kg/d maintains fluid balance in the average adult
- An alternate method of determining fluid requirements is
to provide 1 mL/kcal/d.
- In general, fluid gains (intake) should be in balance with
fluid losses (output).
- Excess fluid administration and/or decreased urine output
contribute to fluid overload
- Fluid losses can be influenced by diarrhea, wounds,
surgical drains, nasogastric tube drainage, chest tubes, ostomy output,
pancreatic secretions, and urine output, which can increase fluid requirements
- In advanced liver disease, fluid requirements vary greatly
depending on the patient’s volume status.
- Fluid restriction
of 1.5 L/d may be indicated if there is coexistence of ascites and significant
hyponatremia, typically <120-125 mEq/L.
- Fluid restriction
in cirrhosis is controversial and is not
recommended for those patients with ascites who are hypovolemic or have only
mild to moderate hyponatremia.
NUTRITION
CHALLENGES IN THE CIRRHOTIC PATIENT
- Goals of
nutrition interventions in advanced liver disease are to improve overall
nutrition status, maintain lean body mass, minimize fluid retention, and reduce
morbidity and mortality associated with malnutrition
Optimizing Intake to
Meet Nutrient Needs
- Meal frequency and
timing is of upmost importance
in maintaining nutrition status in the cirrhotic patient
- After a single night fast, calorie requirements in
patients with liver disease remain the same as those with a healthy liver.
However, due to the reduced storage
capacity for glycogen, the fuel sources being used by cirrhotic patients after a 10-hour fast are the same as what would be used after a 3-day fast in a patient with a healthy
liver
- The reduction in hepatic glycogen stores causes an
increase in use of muscle glycogen, free fatty acid oxidation, and hepatic
production of ketone bodies. The cascading
effects of these metabolic alterations coupled with poor nutrition intake lead
to rapid loss of lean body mass and wasting.
- The prevention
of long periods of fasting may
lessen the muscle loss seen in the cirrhotic patient
- The intake of 4-6
small portion-sized meals daily is generally recommended to promote protein-calorie intake, prevent
longer periods of fasting, and have a muscle-sparing effect.
- The addition of a bedtime or late evening nutrient-dense
snack or nutrition supplement
has been shown to increase total body protein levels in both
decompensated and well-compensated cirrhosis
- Importantly, in the hospital setting, patients are often
kept fasting for testing and may be away from the floor during mealtimes. Infusion
of intravenous (IV) fluids
containing dextrose at the rate of 2-3 g/kg/d may be beneficial in preventing hypoglycaemia and overuse of muscle
glycogen stores if the patient will be
fasting for >10 hours
Protein
Supplementation and Hepatic Encephalopathy
- The use of serum
ammonia levels as an indicator to initiate protein restriction is discouraged
because of a lack of correlation between ammonia and encephalopathy and the
high protein needs of the cirrhotic patient previously described
- The role of
branched-chain amino acids (BCAAs)
and aromatic amino acids (AAAs) in
liver disease has long been studied and debated
- Amino acid metabolism is altered in hepatic failure and
leads to an increase in circulating levels of AAAs (phenylalanine, tyrosine,
and tryptophan) and a decrease in levels of BCAAs (isoleucine, leucine, and
valine)
- It was hypothesized
that the increase in AAAs is a
contributing factor in hepatic encephalopathy (HE) as AAAs can cross the
blood-brain barrier and act as false neurotransmitters
- On this basis, it was speculated
that a modification of the ratio of
dietary AAA to BCAA would help in
the management of HE while allowing sufficient protein intake
- Subsequent studies,
however, have not supported this
approach, finding that BCAA supplementation, although reducing plasma
concentrations of AAA, does not consistently improve encephalopathy
- Nevertheless, recent studies suggest that the use of BCAAs in advanced cirrhosis may have other benefits
- Although no significant improvement in encephalopathy, but
improvements in overall nutrition status, including increased serum albumin and
improved energy metabolism, were noted in the BCAA group compared with the
control group
- Other potential benefits of BCAA supplementation reported
include a decrease in frequency of hospitalizations, improved Child-Pugh score,
and improved overall quality of life.
- When used as a bedtime snack, BCAA supplements were found
to be more beneficial than regular food at improving serum albumin and
preventing muscle catabolism.
- Compliance with long-term use of BCAA may be problematic
due to poor palatability. BCAAs in the form of granules may be better accepted.
- Glutamine may
promotes muscles development. However, it is metabolized into ammonia and may
increase plasms ammonia levels in cirrhotic patients. Therefore, despite the
unclear significance of an elevated ammonia level, it may be advisable for
cirrhotic patients to avoid
glutamine supplements until more information is available
- Prebiotic,
probiotic, and synbiotic (combination of prebiotic and probiotic agents)
agents are currently being evaluated in the treatment of nonsevere HE.
- Two recent RCTs involving patients with HE showed a
reduction in serum ammonia levels plus an improvement in protein tolerance and
neurological symptoms in the symbiotic/probiotic group [Study 1: Bifidobacterium
species + fructo-oligosaccharide (FOS); Study 2: probiotic yogurt without
FOS]
- Although additional
investigation is needed, these studies showed improvements with the probiotic
supplements plus FOS that were equal to those of lactulose therapy, the
general standard of care treatment of HE, with fewer side effects, better patient
compliance, and beneficial effects that lasted even when there was an
interruption in treatment
- The use of pre- and probiotics in this setting may,
therefore, offer a more tolerable treatment to lactulose and a less expensive,
lower risk alternative to antibiotic therapy. Further studies on this subject are
clearly warranted.
- Among liver
transplant recipients, prebiotics
and probiotics may also offer protection
from infection.
- A significant reduction in postoperative infection rates
was seen in the group who received synbiotic-containing formula than group with
standard fiber-free formula, in a recent study of liver transplant recipients
with early EN.
Nutrition Support
- The long-term
benefits of nutrition support in liver cirrhosis remain controversial
- If nutrition support is initiated early, improvement in
mortality may be seen.
- However, since malnutrition is often diagnosed later in
the course of the disease, it may be difficult to demonstrate improvements in
overall morbidity and mortality
- Conversely, other studies show that continuous or cyclical
nutrition support may be considered to supplement
the oral diet as early and aggressive nutrition interventions have been
shown to improve morbidity and mortality if oral intake is insufficient
Enteral Nutrition (EN)
- EN is the preferred route for nutrition support when the
GI tract is functional.
- The European Society for Parenteral and Enteral Nutrition
(ESPEN) guidelines recommend initiating EN if energy intake does not meet estimated
energy needs to improve nutrition status or slow the rate of decline.
- EN may be initiated
via a fine-bore nasoenteral tube, even
in patients with nonbleeding esophageal
varices.
- The placement of a percutaneous
gastrostomy tube (either endoscopically or radiologically placed) is
generally not recommended in patients
with ascites or gastric varices given the potential for ascitic fluid
leakage, infection, and bleeding.
- Aspiration
precautions should be taken when the feeding is into the stomach due to an
increased presence of gastroparesis in the cirrhotic patient.
- An alternative is to advance
the tube beyond the pylorus and feed into the small bowel
- Routine use of a
BCAA-enriched “hepatic” enteral formula is not necessary in advanced liver
disease
- The use of immune-enhancing
formulas remains controversial,
and further well-designed clinical trials are needed
- A concentrated,
high-energy formula (≥1.5 kcal/mL) is
preferred in patients with hyponatremia
and ascites to regulate fluid balance
- In addition, a calorie-dense formula may be better
tolerated because it can be delivered at a low rate and still allow for adequate
provision of nutrients.
- In hepatorenal
syndrome, a renal (low-electrolyte)
formula may be indicated when persistent hyperkalemia and/or hyperphosphatemia
are present
Parenteral Nutrition (PN)
- PN should be reserved for those patients who do not
tolerate EN.
- EN may not be tolerated in advanced cirrhosis because of the presence of severe
ascites or other factors such as nausea, bloating, abdominal distension, and
discomfort.
- Occasionally, EN
may not be possible in patients with advanced cirrhosis due to hemodynamic
instability from a decrease in systemic vascular resistance that may require
vasopressors
- When PN is used,
glucose levels should be monitored carefully due to an increased
likelihood of glycemic imbalance. If hyperglycemia
develops, the amount of dextrose
should be reduced to 2-3 g/kg/d.
- PN may need to be concentrated
to prevent fluid overload
- In patients with cholestasis,
reductions in manganese and copper
should be considered due to impaired biliary excretion of these trace elements.
- The composition
of dextrose and fat should generally
be balanced to decrease the
incidence of steatosis.
- In patients receiving long-term
PN, parenteral lipid emulsion
should not provide >1 g/kg/d to
reduce any further risk of worsening the liver disease
- A cyclic PN
infusion regimen allows a period of time off PN and has a favorable effect on serum liver enzymes
compared with continuous PN infusion
- Liver tests and electrolytes
should be monitored regularly in patients receiving long-term PN, especially those with preexisting liver disease.
- PN may lead to
hepatic steatosis, cholestasis, and eventually fibrosis and cirrhosis.
- When liver tests are elevated in the parenterally fed patient,
and it is not due to medication or disease process, adjustments to the PN
regimen should be made, including a reduction in dextrose, fat, and overall
calories (as appropriate), cycling the infusion and/or maximizing use of EN.
Fluid Imbalance
- Sodium levels are often abnormal in the cirrhotic patient
due to complex fluid abnormalities.
- In well-compensated patients, sodium levels are usually
normal.
- As liver disease progresses, the presence of portal hypertension leads to the development of peripheral edema and ascites.
- The movement of fluids
into the extremities and peritoneal cavity leads to decreased renal blood flow
- This change triggers
a decrease in renal sodium and fluid
excretion with an increase in renal
reabsorption to maintain vascular blood volume and blood pressure.
- The overall effect is greater free water accumulation than
sodium retention, leading to dilutional
hyponatremia
- Body sodium levels may be further depleted due to urinary
losses from high-dose diuretics, GI tract losses, and/or decreased energy
intake.
- Increased fluid intake due to polydipsia can further
exacerbate hyponatremia
Ascites
- Ascites is the accumulation of fluid in the peritoneal
cavity and typically occurs in patients with advanced cirrhosis.
- The presence of ascites has been correlated with a decline
in nutrition status, and those with tense ascites typically have the lowest protein-energy
intake as well as the most compromised nutrition status.
- Aqel et al evaluated gastric function and symptoms in
cirrhotic patients with ascites both before and after large-volume
paracentesis. After paracentesis, patients reported an improvement in early
satiety and were able to consume larger meals. These changes were found to
correlate with enhanced gastric accommodation.
- An important nutrition goal is to reduce the amount of
ascites when present and promote protein-energy intake
- Ascites is generally managed
initially with sodium restriction, usually
≤2
g sodium per day, and diuretics.
- This strategy has been shown to be effective in more than
90% of patients in achieving a reduction in the volume of ascites to acceptable
levels.
- Fluid restriction
(1-1.5 L) is not necessary in
treating patients with ascites unless
the serum sodium is <120-125 mEq/L.
- Fluid restrictions should be recommended with care due to
frequency of plasma hypovolemia seen in conjunction with ascites.
- Refractory ascites
is defined as ascites that is either unresponsive to diuretics and a low-sodium
diet or is resistant to the use of diuretics due to the development of severe
electrolyte or renal impairment
- Refractory ascites often requires repeated large-volume paracentesis; however, paracentesis removes not only fluid from the
peritoneal space but also a large amount
of calories in the form of carbohydrates, proteins, and fats.
- As a consequence, the frequent need for large volume
paracentesis can further advance the
catabolic state already seen in advanced cirrhosis
- This calorie loss needs
to be replaced to prevent further decline in nutrition status.
- It is a common practice to administer a serum albumin infusion after paracentesis when >5 L of
fluid is removed to promote plasma
expansion and to prevent hyponatremia and renal insults.
- Importantly, the use of serum albumin in this setting has
no effect on nutrition status.
- A late evening
snack or post-paracentesis PN was demonstrated to improve morbidity and mortality compared with a control group
receiving a low-sodium diet that provided 30-35 kcal/kg daily.
- Even so, the administration
of PN is not recommended after large-volume paracentesis to replete
nutrition losses
Hepatorenal Syndrome
- Hepatorenal
syndrome (HRS) is an oftentimes fatal complication occurring in acute and
chronic liver failure.
- It is characterized by functional renal failure due to renal vasoconstriction in the absence
of underlying kidney pathology.
-There are 2 types
of HRS: type 1 progresses rapidly and generally has poor prognosis, whereas
type 2 has a slower onset and progression.
- In appropriate candidates, liver transplantation will typically
correct the renal failure without the need for a concomitant renal transplant.
- Nutrition therapy for HRS generally consists of a low-salt diet along with fluid restriction in
those with hyponatremia.
- In some instances, HRS may lead to chronic kidney damage
and may require renal replacement therapy.In these cases, a renal diet may be
warranted if potassium and phosphorus levels become elevated.
Obesity
- There has been much debate regarding assessing energy
needs in the obese patient.
- In patients with nonalcoholic fatty liver disease (NAFLD)
who are clinically well compensated
but have advanced fibrosis, weight
reduction becomes an urgent issue.
- Obese patients with NAFLD-related decompensated cirrhosis are often significantly malnourished with
protein deficits due to the catabolic nature of cirrhosis. In these patients, weight loss remains beneficial;
however, it is important to ensure
adequate protein needs are being met
Insulin Resistance
and Diabetes
- Chronic liver disease patients have a high prevalence of
glucose intolerance and overt diabetes due to the presence of insulin
resistance and β-cell
dysfunction.
- Liver disease may somehow be the initiating factor in the
development of diabetes if the cirrhotic patient presents with 1 or more of the
following conditions: the diagnosis of cirrhosis was established at least 5
years before the onset of diabetes; there is no family history of diabetes; the
patient lacks any factors known to adversely affect glucose metabolism such as
obesity, excessive energy intakes, or medication side effects; and the patient
has no history of hemochromatosis or exocrine pancreatic dysfunction
- Interestingly, there is a positive association between
ferritin concentrations and insulin resistance. A recent study demonstrated that
phlebotomy led to a decrease in both ferritin level and insulin resistance
- Type 2 diabetes mellitus (T2DM) is strongly associated with
NAFLD
- Both hepatic fat and visceral obesity correlate with
insulin resistance, an important precursor in the development of T2DM.
- Metabolic changes are seen not only in fatty liver disease
but also in many other diseases of the liver, including chronic hepatitis C
(HCV) and cryptogenic cirrhosis. The prevalence of diabetes in HCV is 2-3 times
higher than the general population
- Due to the high prevalence of impaired glucose metabolism
and the important role of the liver in carbohydrate metabolism, it is generally
assumed that impaired hepatic metabolism plays a major role in the cause of
impaired glucose tolerance in liver disease. However, it has been demonstrated that
insulin resistance also resides in the muscle, which makes the role of the
liver less clear
- Dietary and lifestyle interventions to promote weight reduction
can help improve glycemic control, insulin resistance, and the lipid profile
while reversing histological changes seen in liver disease
- Gradual weight
reduction is recommended in patients with diabetes with a BMI >25
kg/m2, with a goal weight loss of at
least 5%-10% to improve insulin sensitivity
- Creating a calorie deficit of 500-1000 kcal/d should
result in a modest rate of weight loss
- The maintenance of
high caloric intake is advocated especially during periods of hospitalization
or illness; promoting weight loss during these times may not be warranted.
- The use of bariatric surgery or weight loss medications
may be useful but should be used with caution as in even the well-compensated cirrhotic,
rapid weight loss may cause decompensation
CONCLUSION
- The relationship between malnutrition and survival in
patients with advanced liver disease is complex and multifactorial
- Malnutrition is a common complication of cirrhosis, and
its presence carries with it important prognostic implications. In addition, a
direct correlation exists between the progression of liver disease and the
severity of malnutrition
- Due to the prevalence of malnutrition and the potential for
serious consequences in the setting of advanced liver disease, nutrition
screening, assessment, and intervention are critical facets in improving
clinical outcomes in these patients.





It was wondering if I could use this write-up on my other website. I will link it back to your website though.Great Thanks.
ReplyDeleteMedical Nutrition Therapy